Prevention of moderate and severe ovarian hyperstimulation syndrome: Role of cetrorelix
Introduction to ovarian hyperstimulation
Ovarian hyperstimulation syndrome (OHSS) is a severe and potentially life-threatening complication that may develop during the early luteal phase or early stages of pregnancy after undergoing in vitro fertilization (IVF) treatment 1. OHSS is among the most frequent complications of IVF, with a prevalence of 30% 2. It results from an exaggerated ovarian response to stimulation and can cause significant clinical concerns during the post-fertilization period. The primary cause of OHSS is excessive vascular permeability due to vascular endothelial growth factor (VEGF) overproduction by granulosa and theca cells during the follicular phase. Other vasoactive substances, such as interleukins and angiotensin II, also enhance vascular permeability, while genetic factors may also predispose individuals to a higher risk1. Additionally, factors such as age, polycystic ovary syndrome, low body mass index, high antral follicle count, increased anti-Mullerian hormone levels, and elevated serum estradiol concentrations are recognized as risk factors for OHSS 3.
Effective strategies for preventing OHSS are essential to minimize risks and improve patient outcomes in IVF/intracytoplasmic sperm injection (ICSI) treatments. Key measures include counselling high-risk patients, utilizing gonadotropin-releasing hormone (GnRH) antagonists and agonists, and implementing individualized gonadotropin dosing 4.
Cetrorelix: The key to effective OHSS management
Cetrorelix, a GnRH antagonist, has been shown to effectively mitigate the risk of OHSS in patients undergoing IVF. It works by competitively binding to GnRH receptors, thereby preventing GnRH from interacting with its receptors 5. Furthermore, Table 1 highlights the differences in the mechanisms of action, risk profiles, and reversibility of ovarian function of cetrorelix and GnRH agonists 5. Compared to other GnRH antagonists such as ganirelix, cetrorelix has the added advantage of being effective in both long and short protocols. Additionally, it can lower VEGF secretion, which plays a central role in the pathogenesis of OHSS. By controlling vascular permeability, cetrorelix helps prevent the development of OHSS 6. Furthermore, Table 1 highlights the differences in the mechanisms of action, risk profiles, and reversibility of ovarian function of cetrorelix and GnRH agonists 5.
| Table 1. A brief comparison of cetrorelix vs. GnRH agonist5 | ||
| Criteria | Cetrorelix | GnRH agonists |
| Onset of action | Immediate | Delayed (flare-up effect) |
| Risk of OHSS | Low (minimal risk for OHSS) | Higher risk of OHSS |
| Mode of action | Competitive binding to GnRH receptors | Down-regulation and desensitization of GnRH receptors |
| Reversibility of ovarian function | Immediate | 6 weeks after cessation |
| GnRH, Gonadotropin-releasing hormone; OHSS, Ovarian hyperstimulation syndrome | ||

Clinical efficacy of cetrorelix in OHSS prevention
A prospective case-control study conducted by Vineet et al. investigated the effect of cetrorelix in preventing and treating OHSS in IVF-embryo transfer patients at risk, using both long and short protocols. The study included 102 patients undergoing controlled ovarian stimulation for IVF/ICSI. Patients were divided into two groups: the cetrorelix group, which received cetrorelix 0.25 mg for 5 days, and the control group, which received standard conservative and supportive management for OHSS, including IV fluids, albumin, and paracentesis if OHSS symptoms developed. The data revealed a marked difference between the two groups, particularly in terms of OHSS severity.
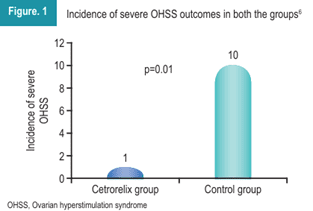
As illustrated in Figure 1, the incidence of severe OHSS was significantly lower in the cetrorelix group compared to the control group.
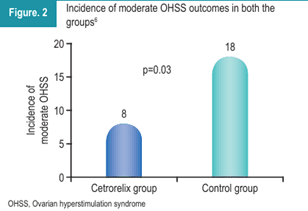
Moderate OHSS was also significantly less frequent in the cetrorelix group (Figure 2). Notably, none of the patients in either group developed critical OHSS.
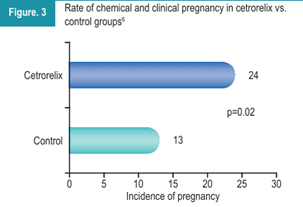
As shown in Figure 3, the pregnancy rate (chemical and clinical) was statistically higher in the cetrorelix group compared to the control group 6.
“The administration of cetrorelix in the early luteal phase of IVF significantly reduced the incidence of moderate and severe OHSS, demonstrating its efficacy and safety as a preventive strategy 6.”
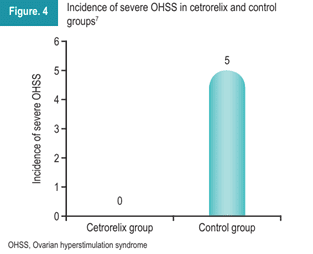
Another retrospective analysis was conducted to investigate the effects of cetrorelix during the early luteal phase on OHSS in patients undergoing IVF embryo transfer. The study included 162 patients who underwent IVF/ICSI. The patients were divided into the cetrorelix group (n=82) and a control group (n=80). After ovum pick-up, the cetrorelix group received 0.25 mg for three days. As depicted in Figure 4, the incidence of severe OHSS was 0 in the cetrorelix group compared to the control group. These results demonstrated that the administration of cetrorelix in the early luteal phase lowered the incidence of severe OHSS. Additionally, it served as a safe and effective approach for preventing OHSS 7.
Summary
OHSS remains a significant complication, highlighting the need for effective preventive strategies in IVF treatments. The use of GnRH antagonists, particularly cetrorelix, has demonstrated substantial efficacy in reducing the incidence and severity of OHSS. Cetrorelix works by competitively inhibiting GnRH receptors, which mitigates excessive ovarian stimulation, lowers VEGF secretion, and controls vascular permeability. Clinical studies have demonstrated that cetrorelix significantly reduced the incidence and severity of OHSS, making it an effective preventive treatment while improving pregnancy outcomes in at-risk IVF patients.
References
- Palomba S, Costanzi F, Nelson S, et al. Interventions to prevent or reduce the incidence and severity of ovarian hyperstimulation syndrome: A systematic umbrella review of the best clinical evidence. Reprod Biol Endocrinol. 2023;21(1).
- Gullo G, Cucinella G, Stojanovic V, et al. Ovarian hyperstimulation syndrome (OHSS): A narrative review and legal implications. J Pers Med. 2024;14(9):915.
- Sun B, Ma Y, Li L, et al. Factors associated with ovarian hyperstimulation syndrome (OHSS) severity in women with polycystic ovary syndrome undergoing IVF/ICSI. Front Endocrinol. 2021;11:615957.
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@ asrm.org. Prevention of moderate and severe ovarian hyperstimulation syndrome: A guideline. Fertil Steril. 2024;121(2):230–45.
- Findeklee S, Diedrich K. Cetrorelix in reproductive medicine. F&S Reports. 2023;4(2):62–4.
- Mishra V, Rane P, Aggarwal R, et al. Role of cetrorelix in the prevention and treatment of ovarian hyperstimulation syndrome: A prospective case control study. IJRCOG. 2023;12(11):3252–6.
- Chen C, Liu L, Geng Y, et al. Prevention and treatment of OHSS by administration of GnRH antagonist in the early luteal phase of controlled ovarian hyperstimulation cycles. Int J Clin Exp Pathol. 2017;10(2):2193–98.






Comments: