Cetrorelix in GnRH-antagonist protocol: Effect on outcome of COH, ET and LH changes
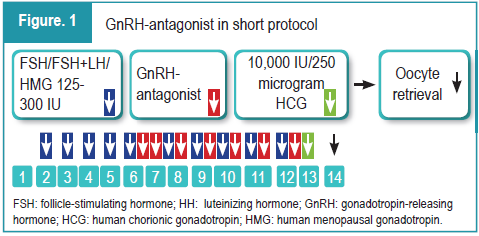
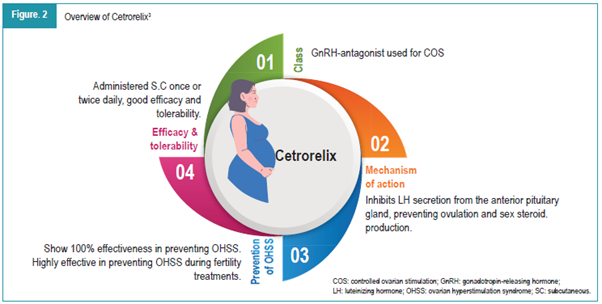
Controlled ovarian hyperstimulation (COH) is a key component of assisted reproductive technology (ART), aimed at inducing the development of multiple follicles to increase the chances of successful invitro fertilization (IVF) outcomes.1 Traditionally, gonadotropin-releasing hormone (GnRH) agonists have been used to prevent premature luteinizing hormone (LH) surges during stimulation, but the introduction of GnRH-antagonists has provided a more tailored approach to this process.2 Cetrorelix, a third-generation GnRH-antagonist, offers several advantages over agonists, such as a shorter treatment duration and reduced risk of ovarian hyperstimulation syndrome (OHSS). By suppressing endogenous LH production, Cetrorelix helps in controlling premature LH surges, which could otherwise affect the follicle development, egg maturation, and ultimately embryo quality and implantation potential.3 The course of the medication depending on the cycle day in ovarian stimulation with the short antagonist protocol is illustrated in Figure 1. The properties of Cetrorelix are elaborated in Figure 2.3
Cetrorelix in GnRH-antagonist protocol: Effects on COH, ET and LH changes3
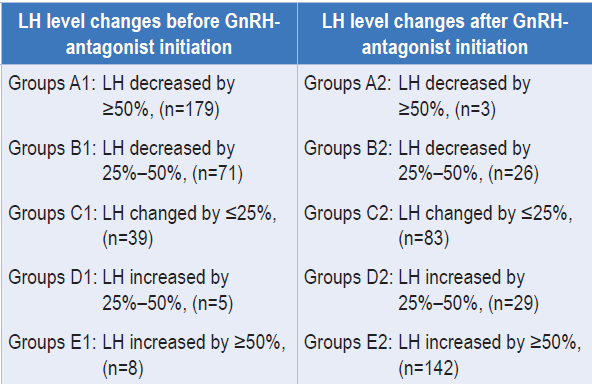
In a study, researchers assessed the effect of LH level fluctuations on the outcomes of COH and embryo transfer (ET) within GnRH-antagonist protocol (cetrorelix).4 A retrospective analysis was conducted on 721 patients undergoing their first IVF/ICSI cycle with the GnRH-antagonist protocol. The COH process was divided into two stages: before (stage 1) and after
(stage 2) GnRH-antagonist initiation. Patients were grouped based on LH level changes in both stages:
Effect of LH level changes before initiation of GnRH-antagonist on the outcome of COH and ET4
The duration of stimulation and total dose of gonadotropins across the five groups showed no significant differences, but Group D1 had a lower total dose and shorter duration of GnRH-antagonist use (p < 0.05).
- Significant differences in FSH and E2 levels were observed among all groups during ovarian stimulation (p < 0.05).
- No statistical differences were found in progesterone levels or endometrial thickness on the HCG trigger day across the groups (p > 0.05).
- LH changes before GnRH-antagonist initiation had no significant correlation with COH outcomes across all groups (p > 0.05).
- ET outcomes were similar among the groups (p > 0.05).
Effect of LH level changes after initiation of GnRH-antagonist on the outcome of COH and ET4
Group A2 had a shorter ovarian stimulation duration and a lower total dose of gonadotropins compared to the other groups (p=0.004, p < 0.001).
Additionally, FSH levels in Group A2 were the lowest on both the initiation day of stimulation and the HCG trigger day (p < 0.001).
In Group A2, the number of oocytes retrieved, mature oocytes, 2PN fertilized oocytes, cleaved embryos, and available embryos were significantly higher than in the other groups (p < 0.05).
- Group A2 also had the highest cycle cancellation rate (77.0%) (p < 0.001), but no significant differences ET outcomes were found across all groups (p > 0.05).
- LH level changes after antagonist initiation did not significantly affect the number of oocytes retrieved. Additionally, patient age, AMH, AFC, and LH changes had no significant impact on the number of available embryos.
- While LH level changes after antagonist initiation had no significant effect on clinical pregnancy outcomes, patient age had a significant effect on clinical pregnancy rates
Results indicated no significant differences among groups in stage 1 regarding COH and ET outcomes. However, in stage 2, a greater decrease in LH levels was associated with higher numbers of retrieved oocytes, mature oocytes, fertilized oocytes, cleaved embryos, and available embryos (p < 0.05). Interestingly, no significant differences in ET outcomes were observed.
Additionally, the freeze-all rate was significantly higher in Group A2 (p < 0.001). In conclusion, LH changes before the addition of GnRH-antagonist were unrelated to COH and ET outcomes, while changes following GnRH-antagonist initiation significantly affected COH outcomes, but not ET results.4
For LH changes with addition of GnRH protocol with cetrorelix are long lasting as the terminal half-life of cetrorelix is 30 hours after subcutaneous injection, resulting in duration of cetrorelix plasma concentration for longer than 20 hours. In a study, researchers reported an average duration of cetrorelix injection to be 3.82 ± 1.44 days. These doses ensured that, for patients without risk of premature LH surge, the cetrorelix levels in the plasma remained abundant to suppress LH levels.5
Summary
- Cetrorelix, a third-generation GnRH-antagonist,offers key advantages over agonists, Including shorter treatment duration and reduced risk of OHSS.
- By suppressing endogenous LH production, Cetrorelix prevents premature LH surges, which are crucial for maintaining optimal follicle development, egg maturation, and embryo quality.
- The GnRH-antagonist protocol with cetrorelix is widely used in ART due to its ability to rapidly and effectively inhibit LH surges, avoid flare-up effects, reduce OHSS incidence, and shorten the treatment period.
- LH is a glycoprotein hormone critical for hormone production, ovulation, and luteinization. During COH, LH levels typically decrease before antagonist , although some patients experience an increase.
- Both excessively high or low LH levels, and significant changes in LH, can negatively affect clinical pregnancy rates, with decreased LH levels yielding better COH outcomes than increased levels.
- While LH changes before GnRH-antagonist initiation have no eff ect on COH and embryo transfer outcomes, LH changes after antagonist initiation significantly affect oocyte retrieval, fertilization, embryo quality, and availability, though they do not impact pregnancy outcomes.
- Therefore, LH level changes during the GnRH antagonist protocol can serve as indicators for COH outcomes, but are not reliable predictors of clinical pregnancy results.
References
- Yang H, Zheng C, Zheng Q, et al. Controlled ovarian hyperstimulation for poor ovarian responders undergoing in vitro fertilisation/intracytoplasmic sperm injection: a protocol for systematic review and Bayesian network meta-analysis. BMJ Open. 2021;11(2):e039122.
- Kadoura S, Alhalabi M, Nattouf AH. Conventional GnRH antagonist protocols versus long GnRH agonist protocol in IVF/ICSI cycles of polycystic ovary syndrome women: a systematic review and meta-analysis. Sci Rep. 2022;12(1):4456.
- Findeklee S, Diedrich PK. Cetrorelix in reproductive medicine. F S Rep. 2022;4(2 Suppl):62-64.
- Zhou JS, Chen JH, Tang FF, Ou JP, Tao X, Cai LH. The eff ect of luteinizing hormone changes in GnRH antagonist protocol on the outcome of controlled ovarian hyperstimulation and embryo transfer. BMC Pregnancy Childbirth. 2023;23(1):604.
- Xu H, Zhao S, Gao X, et al. GnRH antagonist protocol with cessation of cetrorelix on trigger day improves embryological outcomes for patients with sufficient ovarian reserve. Front Endocrinol (Lausanne). 2021;12:758896.






Comments: